21 CFR Part 11
21 CFR Part 11 is the FDA’s regulations for electronic documentation and electronic signatures. It serves as an outline for the administration of electronic records in a pharmaceutical company’s quality management system.
The 21 CFR Part 11 regulations were first published back in 1997, and since then electronic systems and their capabilities have advanced remarkably. Even with all the technological advancements, the purpose of 21 CFR Part 11 still remains the same over two decades later.
Part 11 was designed to cater to the evolving needs of the pharmaceutical industry, with the purpose of helping companies:
- Properly Utilize computer systems and software.
- Maintain data security to ensure it is not lost or corrupted.
- Ensure the integrity of approval and review signatures.
- Enable the tracing of changes to any data.
- Prevention and detection of falsified records.
Electronic records have become commonplace in the industry, with almost every pharmaceutical production facility requiring 21 CFR Part 11 Compliance. Many manufactures have Nikka Densok HVLD equipment that predates the 21 CFR Part 11 requirement, so in response Nikka Densok USA has developed a 21 CFR Part 11 Upgrade Package to meet this demand. Please see below:
21 CFR PART 11 PACKAGE
Software to meet the demand
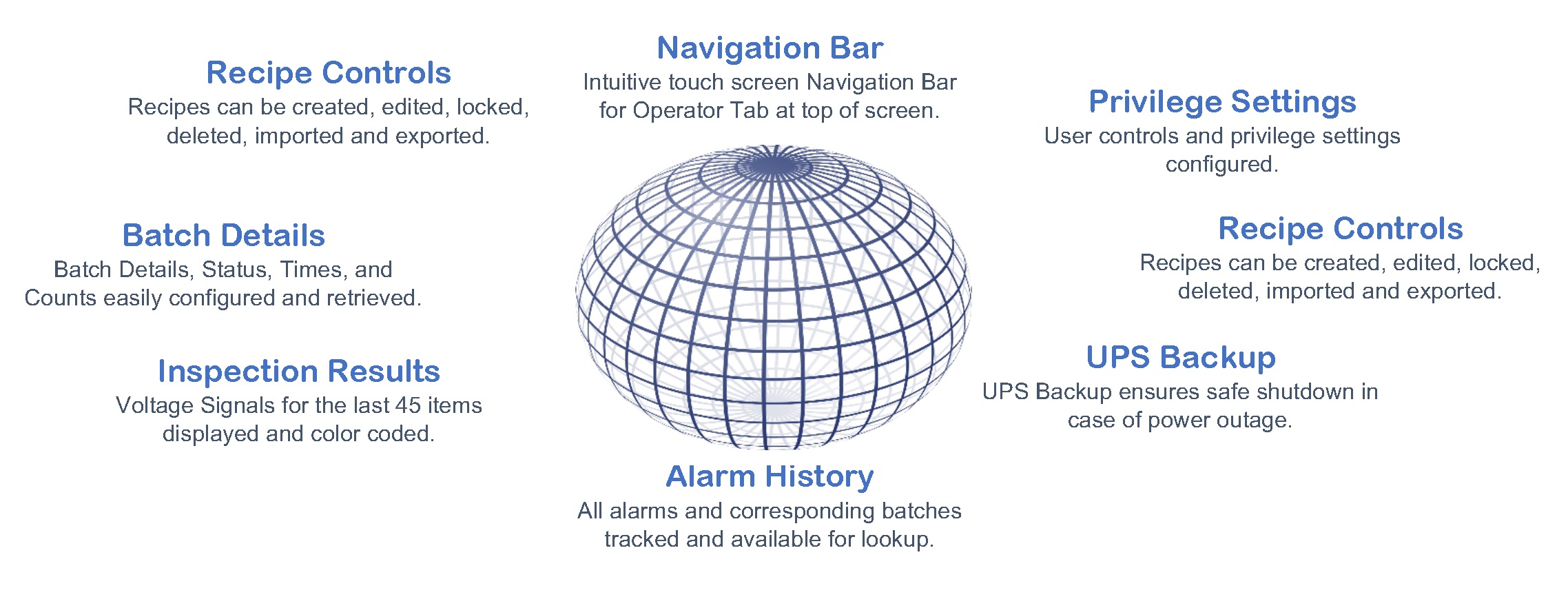
Hardware to enhance the experience
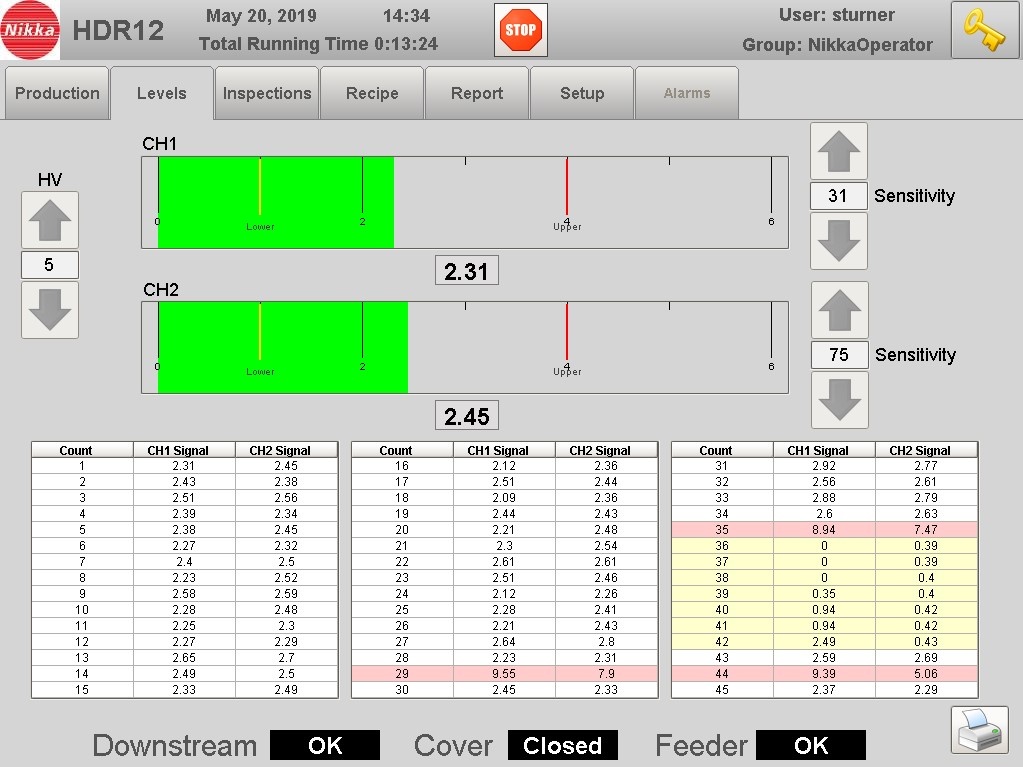
Panel PC HMI
Each 21 CFR Part 11 package utilizes a Pro-face Panel PC as the HMI, providing touch screen capability and an overall enhanced operator experience.
HMI Custom Housing
Included with the Panel PC HMI is a custom fit stainless-steel housing, aesthetically matching the existing machine. Strong Arm upgrade for HMI also available.
UPS Battery Backup
Rail-mounted UPS battery backup to save space and ensure data in case of power outage
Installation Process
Local Installation
All installation technicians are deployed from right here in the USA.
Timeline
Upon the receipt of purchase order, Nikka Densok USA will immediately work with you and your team to determine the optimal installation schedule. A typical installation lasts 3-5 days, and largely depends on the whether the connection is Active Directory (domain or group) versus a Local User. The Nikka Densok USA Project Manager will coordinate timelines and installation expectations with the on-site contact.
Requirements
Nikka Densok USA technicians will need full access to the machine during the installation period and the customer IT department may be required for network access depending on the connection needed. Please ensure all production team members and management is notified that the machine will be off-line during the installation process.
Shipping & Storage
Nikka Densok USA may need to pre-ship materials to the customer location prior to the installation date. If available, please inform us of the on-site secure storage address.
Ongoing Support
Maintenance
The price quoted is inclusive of Nikka Densok USA utilizing remote support to resolve any unforeseen maintenance issues that would affect the performance of the 21 CFR Part 11 software or hardware. Any other machine related issues requiring on-site support are billed per the Nikka Densok USA hourly service and maintenance rate effective 6/1/2020.
Swift Responsiveness
Nikka Densok USA prides itself on responsiveness and vows to respond to any service or maintenance inquiries, or issues, within 24 hours of communication.
Hardware Warranty
The Pro-face HMI, stainless-steel housing and the Phoenix Contact UPS all come with an unlimited 1 year Warranty from date of installation.
